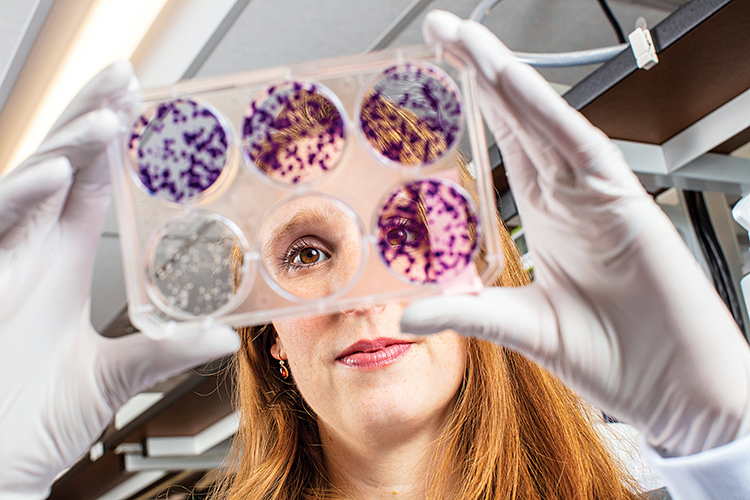
Hilary Nicholson ’12 is using a decades-old concept to take aim at cancer.
On Dec. 10, 2019, as medical oncologist William G. Kaelin Jr. walked across the midnight-blue carpet of the Stockholm Concert Hall to receive the Nobel Prize in Physiology or Medicine, Hilary Nicholson ’12 was watching from the nearby Grand Hotel. Kaelin and two collaborators were being honored for having detailed the molecular mechanism by which all animals sense and adapt to changes in oxygen. But Nicholson wasn’t in Sweden just to applaud her mentor. She was also there to deliver a lecture on how she was using Kaelin’s discovery to develop new, more precise ways to treat kidney cancer.
This year, an estimated 73,750 Americans will be diagnosed with kidney cancer; some 14,820 of them will die from it, according to the American Cancer Society. Nicholson, a cancer biologist, focuses on the most common form of the disease: clear cell renal cell carcinoma, or ccRCC, which accounts for more than 70% of all adult cases.
In a paper published in Science Signaling on Oct. 1, 2019 — six days before Kaelin got the call from the Nobel committee — Nicholson and her coauthors reveal a possible new target for treating kidney cancer. Central to their findings is the pathway Kaelin illuminated, which involves the interaction between two proteins, HIF and VHL. HIF causes new blood vessels to grow when needed, “which is great if you’re a mountain climber at 8,000 feet,” says Nicholson — and also if you’re an oxygen-hungry cancer cell. VHL, a tumor suppressor, destroys HIF when it senses a cell’s got enough oxygen. In 90% of ccRCCs, though, VHL is mutated — so more and more blood vessels are produced, enabling cancer cells to thrive.
Nicholson’s approach, she says, was “to find a way to exploit this mutation and use it against the cancer.”
To do so, she deployed a concept known as synthetic lethality. While it sounds like a good reason not to wear polyester pants, the phrase, coined in the 1940s, actually refers to a relationship between two genes in which a mutation in one has no effect on a cell, but a mutation in both leads to cell death. This approach is particularly attractive in cancer therapy because it makes it possible to target only cells that have the cancer-specific mutation, leaving healthy cells untouched.
In fruit fly and human cells as well as in animal models, Nicholson and her team identified a way to deliver a one-two punch to kidney cancer by combining a drug that is lethal to VHL-mutant cells with an inhibitor of HIF. “That’s hitting on both sides,” she explains, “killing the cancer and preventing it from being able to hijack the blood supply.” The results are so promising that the FDA has already approved a clinical trial based on the study.
After working in Kaelin’s lab at the Dana Farber Cancer Institute for four years, Nicholson took on a new role in January, as a senior scientist at Tango Therapeutics. Established in 2017, the Cambridge start-up is using synthetic lethality to discover new targets for cancer treatment. Kaelin is a cofounder.
Nicholson, a biochemistry major who graduated with high honors, fondly remembers learning from professors Roger Rowlett and Ernest Nolen and says she discovered her passion for research at Colgate. “The thing that I’ve always found so beautiful about science is that it’s the pursuit of the truth,” she says. “At the end of the day, if you conduct a good experiment, if you ask a good question, your answer should be correct. It should prove new truth.”